Put yourself in a world with a harsh, dark, fiery atmosphere. There is no one in sight. Clouds of smoke, chains of lightning and streams of lava cascade across this hellish landscape. The air is hard to breath, toxic and hot.
Where are you?
Here on Earth, about 3.8 billion years ago. It is these conditions that Dr. Stanley Miller and Dr. Harold Urey wanted to recreate in their famous 1953 experiment, but the means by which they did so were dangerous.
Now, a group of researchers from the Georgia Institute of Technology has recreated the basis of the Miller-Urey experiment, this time with a different set-up than the original and with more knowledge of Earth’s pre-biotic conditions, according to a Jan. 21 press release in the Journal of Visualized Experiments.
“We did a more realistic, safer, representation of the experiment,” said Dr. Eric Parker, a graduate student from GIT and a researcher for the study. “My colleagues and I have been approached many times on how to do the Miller-Urey experiment. We have always been hesitant to explain how to conduct the experiment since there is a major risk of explosion while conducting the experiment.”
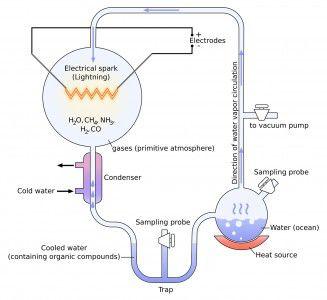
The Miller-Urey experiment is famous for its probe into the origins of life on Earth. In short, Miller and Urey mimicked circumstances similar to those on Earth about 0.7 billion years after it formed, which is fairly young in geological terms.
The original experiment aimed to recreate the chaotic conditions of the young Earth with a two-flask apparatus. One flask was filled with water that is boiled to simulate evaporation. The other flask contains four gases: methane (CH4), diatomic hydrogen gas (H2), ammonia (NH3) and water vapor (H2O).
Both flasks were connected by a series of tubes including a condenser so any gases present in the experiment that come in contact with the condenser would exit the gas phase and enter the liquid phase prior to traveling back to the flask that contained water. This process mirrored rainfall on the early Earth.
An electric current was used to simulate lightning, provided in the original experiment by a Tesla coil that ran all day for a week straight.
After this reaction ran for seven days, a dark brown liquid formed in a collecting tube. When analyzed, some incredible results were found: From simple inorganic molecules, relatively complex organic molecules were formed. Researchers found amino acids such as glycine and aspartic acid, some of the building blocks of life, in the simulated primordial goop.
In other words, the study suggests that components of life arose naturally from a lifeless world.
The original experiment prompted many scientists to do their own versions, and it also paved the way for the rise and growing interest of the prebiotic chemistry field.
“It was such a huge discovery from that era of biology,” said Edward Loechler, Boston University professor of biology. “To put it simply, it’s bloody complex.”
And it is. But with complexity comes confusion and danger.
The gas composition of the original experiment contained methane and hydrogen — both explosive gases — that could have caused harm if ignited by the Tesla coil.
“Miller and Urey ran a risk of running the tesla coil for that long,” Parker said. “Tesla coils are supposed to be turned for specific period of time. It’s not designed for continuous use.”
To improve safety in the recreation, Parker and his team adjusted the electrical use.
“For our experiment, we reduced the voltage output to 30,000 volts from the original experiment’s 60,000 volts,” he said. “We also put the Tesla coil on a timer so it would discharge at hour intervals so it wasn’t continuously running. This ultimately made our experiment run longer than the original’s, but made our experiment safer and more accurately represented a lightning discharge event on the early Earth.
The team also used a different gas mixture including N2 as well as methane, ammonia and water. This combination better simulates what scientists believe the early atmosphere to have been like, particularly after years of widespread volcanic eruptions.
In their final paper, the researchers also gave insight on how to safely evacuate the gas mixtures before the Tesla coil discharged.
“To reduce any ambiguities and confusion, we wrote and published a paper on how to do correctly and safely,” Parker said. “Any interested scientists can now read about it and do it step by step.”
So what does this mean for the future? Now scientists from all over can recreate this historic experiment safely and correctly thanks to the GIT research team. This could instill a resurgence and further intrigue into the field of prebiotic chemistry. And who knows? With more researchers performing the same experiment, perhaps we could discover even new insights into the origins of life.
This is an account occasionally used by the Daily Free Press editors to post archived posts from previous iterations of the site or otherwise for special circumstance publications. See authorship info on the byline at the top of the page.