The Boston University Alzheimer’s Disease Center is taking part in a national clinical study that could slow the rate of decline in patients with Alzheimer’s disease.
The Alzheimer’s Disease Cooperative Study, called NOBLE, investigates the effects of the drug T-817MA on patients ages 55–85 with Alzheimer’s, said Robert Stern, the principal investigator for the Boston branch of the study. Funded by the National Institute of Health and Toyama Chemical Co. in Japan, the study began its second phrase of testing in March 2014.
“The NOBLE study is an exciting new approach,” Stern said. “There has not been an FDA-approved drug for Alzheimer’s disease in over 10 years … The impact on individuals in our society is so staggering. I am really aching for a new drug to be successful.”
Stern said the study is unlike other studies because it targets patients who already have mild to moderate Alzheimer’s disease.
“Most of the other research studies are focusing on much earlier stages of the disease, with the hope that if you intervene early, even before someone has symptoms, you can slow down the disease enough so the symptoms either won’t develop or they will be much slower,” he said. “I felt there was a need for a good research study to offer people who are in the more advanced stages of the disease, not the very earliest.”
Subjects take either a high dosage of the drug, a low dosage or a placebo over the course of about 12–14 months and go to BU Medical Center periodically for cognitive and physical testing, Stern said. The study also provides and pays for transportation to the testing center if needed.
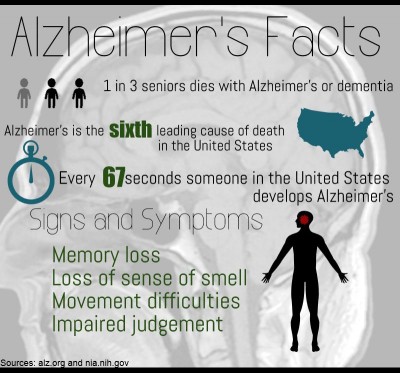
Stern said Alzheimer’s is an abnormal brain disease that affects approximately 5.5 million Americans, and it is not a normal part of aging.
“There’s this belief by both clinicians and the lay public that Alzheimer’s disease is just a normal part of aging and there’s nothing to do. It’s just something that happens.” he said. “All of those things couldn’t be further from the truth.”
The disease can also have widespread economic impacts, including the amount of money spent on Alzheimer’s treatment and prevention, Stern said.
“It is likely the single most important reason why Medicare might go bankrupt in the next couple years,” he said. “One out of four Medicare dollars is spent on Alzheimer’s disease-related problems, and that’s going to grow tremendously over the next several years.”
Jesse Mez, a study physician for the clinical trials, is in charge of conducting neurological evaluations and discussing the risks and benefits with the subjects. He said a drug such as the one from the NOBLE study would be a major breakthrough in the medical field.
“Really the gold mine for Alzheimer’s drugs would be something that will change that rate of decline, and that’s what we hope this medicine will do,” he said. “If this medicine is shown to truly be disease-modifying, then it will be something that neurologists use, that primary physicians use, it’ll be something that’s very important and used widely.”
Mez said he is optimistic but realistic about the outcome of this trial, which will continue until enough subjects are enrolled to determine the drug’s benefits.
“My hope is that we will see benefit and that it will be approved and become part of our toolkit for treating patients with Alzheimer’s disease,” he said. “That being said, many, many drugs have gone to trial and many have reached this stage in terms of clinical research and many have been unsuccessful.”