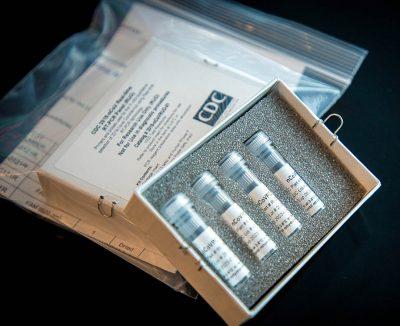
Boston University School of Medicine’s Center for Regenerative Medicine partnered with the Boston Medical Center to develop a clinically approved in-house test for COVID-19. The test was developed and approved within the span of one week by a group of over 50 volunteer researchers on BU’s Medical Campus, and went into use at BMC March 23.
The team at BUSM is headed by professors George Murphy, co-director of CReM, and Christopher Andry, chief of the Department of Pathology and Laboratory Medicine at BUSM and BMC.
Murphy was inspired to launch the development project after hearing from fellow researcher Lea Starita at the University of Washington, according to The Brink — BU’s research-related news publication. Starita had begun enlisting the help of volunteers to develop a coronavirus test at UW due to the lack of commercially available tests in the country, and Murphy recognized that Boston would soon be facing a similar shortage.
In partnership with BMC’s Department of Pathology, Murphy and Andry, along with a team of BUSM researchers, PhD students, post-doctoral fellows and junior faculty, began the development and testing of an in-house coronavirus test.
Aditya Mithal, a PhD candidate at BUSM and researcher at CReM, is a member of the test development team and said the lack of preparedness in the United States to tackle the virus at first set the country on a downward trajectory. He compared the United States’ response to that of South Korea, who’s more immediate testing capabilities alleviated some of the pressure of the disease on the nation.
“South Korea and the United States announced their first COVID-19 case on the same day, and it’s really hard to believe how divergent their paths have been,” Mithal said. “It was clear that more testing was needed [in the U.S.], it was clear that we were not ready as a nation, and private companies had not been developing their own tests that could be rolled out.”
Mithal also said the lengthy process of developing and approving medical tests in the country prevented an immediate response, especially considering the country’s already delayed reaction to the pandemic.
“The amount of tests that are needed are in the tens of millions,” Mithal said, “and to be able to manufacture those and roll them out and get that FDA-approved and be able to ship them out to all fifty states was just very difficult given the time frame in which people started reacting to this pandemic.”
Mithal is one of several BUSM students and researchers whose projects were shut down after being deemed non-essential by President Robert Brown. Once their own research ventures were suspended, Mithal said he and his colleagues began looking for a way to contribute their time and expertise to the coronavirus research.
“We were trying to figure out how we could help and this opportunity presented itself,” Mithal said. “We have a lot of equipment and we have a lot of technical expertise, but obviously we’re a research lab and we’re not set up to do any of this.”
Although CRM was not predisposed for test development, Mithal said the design of the in-house test is familiar to most researchers, and was easy for the BUSM researchers to replicate.
“The test itself is involved in terms of timing,” Mithal said, “but it’s a very standard molecular biology assay that many scientific researchers do all the time, so we knew that we had the expertise to be able to get it done.”
Mithal said the most important element of the test that the researchers focused on was accuracy.
“The biggest nightmare is having an inaccurate test, that’s almost worse than having no test,” Mithal said, “because if you’re telling a clinician this patient is negative when they’re positive, then that’s a disastrous thing.”
In order to deploy the in-house test at BMC, the test first needed to go through the FDA approval process, which Mithal said can take up to a year. To expedite approval, the BUSM lab applied for Emergency Use Authorization, a detailed application through the FDA that allows labs to send interventions and tests into clinical use faster.
Mithal said his team received significant technological and logistical support from the BMC Department of Pathology.
“Getting this all done in a week was quite a herculean task, and we were doing this with full support and collaboration and thanks to the Boston Medical Center Department of Pathology,” Mithal said. “They’re much more familiar with all of the regulatory things that need to happen and they were able to help us work through that whole process. This is all done side by side with them, because this is ultimately a Boston Medical Center test.”
Since the test developed by CReM went live, the research team has been receiving and testing patient samples from BMC everyday.
Mithal said he believes “we’re right at the beginning” of a long period of testing, research development and international cooperation before the coronavirus is contained. He said he believes the next step is the development of a commercially-available antibody base test, which would enable health professionals to identify those who have developed immunity to the virus.
“To be able to understand who is infected and who may potentially develop some sort of immunity to SARS-CoV2 would obviously be really critical,” Mithal said, “to be able to ascertain ways to renormalize societies by allowing people who we know are somewhat immune to be allowed to loosen social distancing restrictions.”
Mithal said the volunteer team of researchers at BUSM knows the pandemic will not be resolved anytime soon, and that the growth of COVID-19 cases in Boston will continue to place pressure on the city’s health systems and testing capabilities. Therefore, Mithal said, they will continue to commit themselves to helping in any way they can.
“We’re just going to chug along here for as long as we need to go,” Mithal said. “We’re here for the long haul, and we’re hoping that we can just try and make a small contribution to keeping Boston safe and healthy.