By Adena Etaat
With more than 1 million people around the world dead from COVID-19, the hunt for a vaccine is driven by the goal to mass-produce a one in 2021 — yet vaccines usually take up to 15 years to develop.
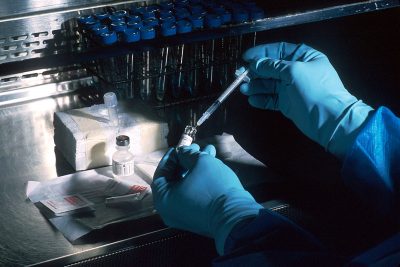
With more than 100 countries aiding its development, the status of a potential vaccine is ever-changing.
Vaccines are derived from weakened parts of the same germ or virus that causes a specific disease. By exposing one’s body to the pathogen, the immune system automatically begins producing antibodies to build immunity to the disease, according to the U.S. Centers for Disease Control and Prevention.
Infectious disease specialist David Hamer, a professor of global health and medicine at Boston University’s School of Public Health, said the COVID-19 vaccine has outpaced previous vaccine developments, which showcases promising progress.
“The whole process of making vaccines to COVID-19 has gone really amazingly quickly,” Hamer said. “We’ve gone from recognizing a new pathogen … less than 12 months ago, and now we have a number of vaccine candidates.”
Hamer said vaccine development typically goes through three phases, during which the medical effectiveness is measured on a number of scales. But, the rush and demand of living in a global pandemic has been expediting the process.
SPH epidemiology professor Matthew Fox said this round of vaccine development is unique in its approach, with some techniques having never been attempted before.
For instance, Cambridge-based Moderna Therapeutics, the first company to enter clinical trials, is steering away from traditional methods of creating an effective vaccine.
Christopher Gill, an infectious disease specialist and SPH associate professor, said vaccines are typically created using protein from the actual virus, but Moderna instead uses the virus’s messenger RNA, which codes for the protein.
“For any vaccine that you can make that follows the classical route, your manufacturing process has to be very specific for that one kind of vaccine,” Gill said. “So in theory, the factory that makes mRNAs for the coronavirus could make mRNAs for anything.”
While the process is more indirect, the manufacturing process has become much more efficient, Gill said.
Healthy volunteers are agreeing to be infected with COVID-19 in trials in the United States and abroad in hopes of further advancing the process.
This desperate position individuals are faced with is one reason the development of the vaccine is progressing so rapidly, Fox said.
“Because we’re in a pandemic situation, it’s much easier to enroll people into trials,” Fox said, “because people are just more willing to put themselves in a trial that’s going to help people.”
The development of the vaccine is progressing, as shown by The New York Times’ Coronavirus Vaccine Tracker, which reports that 11 vaccines have entered Phase 3 large-scale efficacy tests, and six have been approved for early or limited use.
There is a possibility the strain of the virus may evolve, leading to another required vaccine, but Hamer said these mutations will not jeopardize the effectiveness of vaccines currently underway.
Yet, there is faith that COVID-19 is “very stable,” Gill said.
“In a way, the challenge of a COVID-19 vaccine might actually be quite a bit easier than a seasonal flu vaccine,” Gill said, “except for the fact that we’re doing this for the very first time.”
Immunology, the study of immunity, and virology, the study of viruses, are focal points in the coronavirus epidemic, given the still-unclear ways in which the disease can spread.
Other than the chance of mutation, public opposition against vaccines poses a concern for scientists and experts. Hamer said some must be persuaded and reassured that vaccines are safe ways to prevent infectious diseases.
However, negative sentiments against the vaccine may be suppressed, Fox said, once a verified vaccine is distributed.
“If we have a vaccine that is truly safe and reasonably effective, I think people’s minds will change,” Fox said. “I suspect that as the vaccine gets rolled out, again, with the caveat that it’s a safe and reasonably effective vaccine, that we’re going to see that hesitancy change.”
As the end of 2020 approaches and companies worldwide rush to create a vaccine, Hamer said he predicts the deployment of a mass-use vaccine may occur the first quarter of 2021, but more likely the second half of next year.